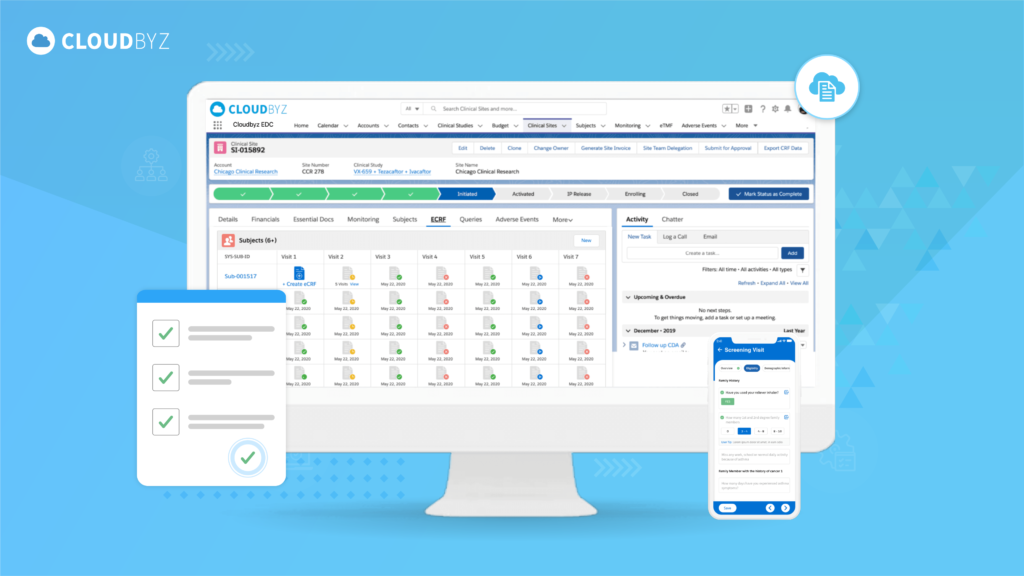
In the rapidly evolving field of clinical research, efficiency and precision are critical. Modern technology plays an essential role in transforming traditional methodologies, enabling more streamlined and accurate processes. Cloudbyz, leveraging the robust Salesforce platform, offers an advanced Electronic Data Capture (EDC) solution designed to accelerate clinical studies, delivering unparalleled benefits to researchers, sponsors, and regulatory bodies.
Understanding Electronic Data Capture (EDC)
Electronic Data Capture (EDC) systems are software solutions used to collect, manage, and store data generated during clinical trials. Unlike paper-based methods, EDC systems provide real-time data entry, reduce manual errors, and enhance the overall efficiency and accuracy of clinical trials. By adopting Cloudbyz EDC, organizations can significantly improve their data management processes and drive faster study completion.
Why Choose Cloudbyz EDC Built on Salesforce?
Salesforce is a leading cloud platform known for its scalability, security, and integration capabilities. Cloudbyz EDC, built natively on Salesforce, takes full advantage of these features, offering a comprehensive solution tailored to the needs of clinical research.
Key Advantages of Cloudbyz EDC:
- Seamless Integration: Cloudbyz EDC integrates effortlessly with other Salesforce applications, such as Cloudbyz Clinical Trial Management Systems (CTMS), and enterprise resource planning (ERP) systems. This integration ensures a unified view of all clinical trial data and activities, enhancing coordination and decision-making.
- Scalability: Salesforce’s cloud infrastructure supports the scalability required for large-scale clinical trials, enabling Cloudbyz EDC to handle extensive data volumes efficiently without compromising performance.
- Security and Compliance: Cloudbyz EDC leverages Salesforce’s robust security features, including data encryption, user authentication, and regular security audits. These features ensure data integrity and compliance with regulatory standards such as FDA 21 CFR Part 11, GDPR, and HIPAA.
- Customization and Flexibility: The Salesforce platform allows for extensive customization. Cloudbyz EDC can be tailored to meet the specific needs of different clinical studies, with custom fields, workflows, and reports, ensuring that the solution fits the unique requirements of each trial.
- Real-Time Data Access and Analytics: Cloudbyz EDC utilizes Salesforce’s powerful analytics tools, providing real-time data visualization and reporting. This capability enables researchers and sponsors to quickly identify trends, monitor progress, and make informed decisions, thereby accelerating the clinical study process.
Benefits of Cloudbyz EDC for Clinical Studies
1. Enhanced Data Accuracy and Quality
Manual data entry is often error-prone, leading to delays and increased costs. Cloudbyz EDC minimizes these errors with user-friendly interfaces, validation rules, and automated data checks. This ensures that the data collected is accurate, complete, and consistent, leading to more reliable study outcomes.
2. Improved Efficiency and Productivity
Traditional data collection methods are time-consuming and labor-intensive. Cloudbyz EDC automates many processes, such as data entry, query management, and reporting, reducing the administrative burden on researchers. This allows them to focus on more critical tasks, such as patient care and data analysis.
3. Faster Study Start-Up and Execution
The cloud-based infrastructure of Salesforce enables quick deployment and configuration of Cloudbyz EDC. This accelerates the study start-up phase, allowing researchers to initiate trials faster. Additionally, real-time data access and seamless integration with other systems facilitate more efficient study execution and monitoring.
4. Enhanced Collaboration and Communication
Cloudbyz EDC promotes collaboration among study stakeholders, including researchers, sponsors, CROs, and regulatory bodies. The platform’s communication tools enable secure data sharing, real-time updates, and collaborative decision-making, ensuring that all parties are aligned and informed throughout the study lifecycle.
5. Streamlined Regulatory Compliance
Compliance with regulatory requirements is crucial in clinical research. Cloudbyz EDC offers built-in compliance features, such as audit trails, electronic signatures, and secure data storage. These features help ensure that the study data meets all regulatory standards, reducing the risk of non-compliance and associated penalties.
Real-World Impact: Success Stories with Cloudbyz EDC
Several organizations have experienced significant benefits by implementing Cloudbyz EDC. For instance, a leading biotech company reduced data entry errors by 30% and decreased study start-up times by 25% after adopting Cloudbyz EDC. Another pharmaceutical firm reported improved data quality and faster regulatory submissions, thanks to the real-time data access and robust analytics provided by the solution.
Conclusion
The Electronic Data Capture (EDC) solution built natively on Salesforce offers a powerful tool for accelerating clinical studies. By enhancing data accuracy, improving efficiency, facilitating collaboration, and ensuring regulatory compliance, Cloudbyz EDC can drive faster, more reliable study outcomes. As the clinical research landscape continues to evolve, leveraging advanced technologies like Cloudbyz EDC will be essential for staying competitive and achieving success in clinical trials.
Embrace the future of clinical research with Cloudbyz EDC and experience the benefits of faster, more efficient, and more accurate clinical studies.


