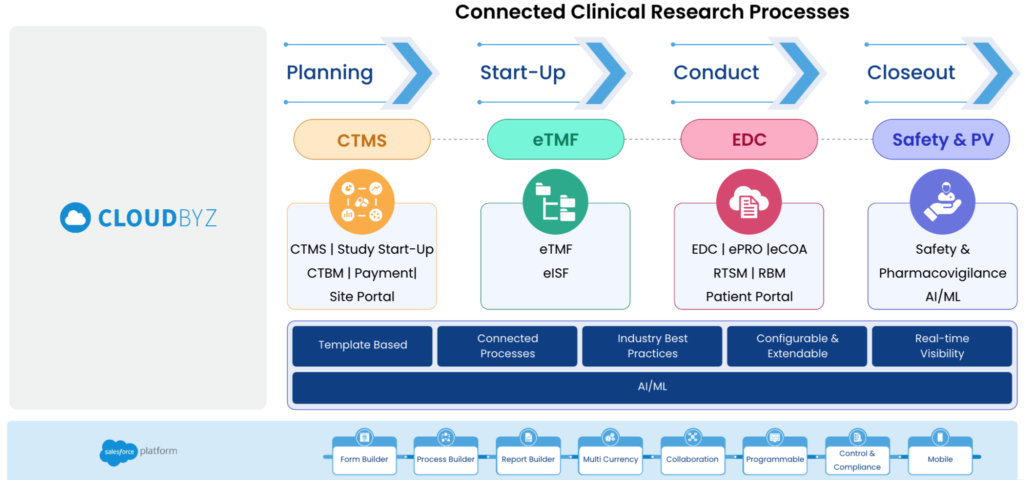
Clinical trials are complex and time-consuming endeavors, requiring efficient management and coordination between various stakeholders. Cloudbyz’s Unified Clinical Trial Management Solution, built on the Salesforce platform, offers a comprehensive, customizable, and secure platform for streamlining clinical trial operations. In this blog post, we will explore the unique value propositions of Cloudbyz’s solution that sets it apart in the clinical trial management software market, along with additional benefits that elevate its position in the industry.
- Comprehensive Solution:
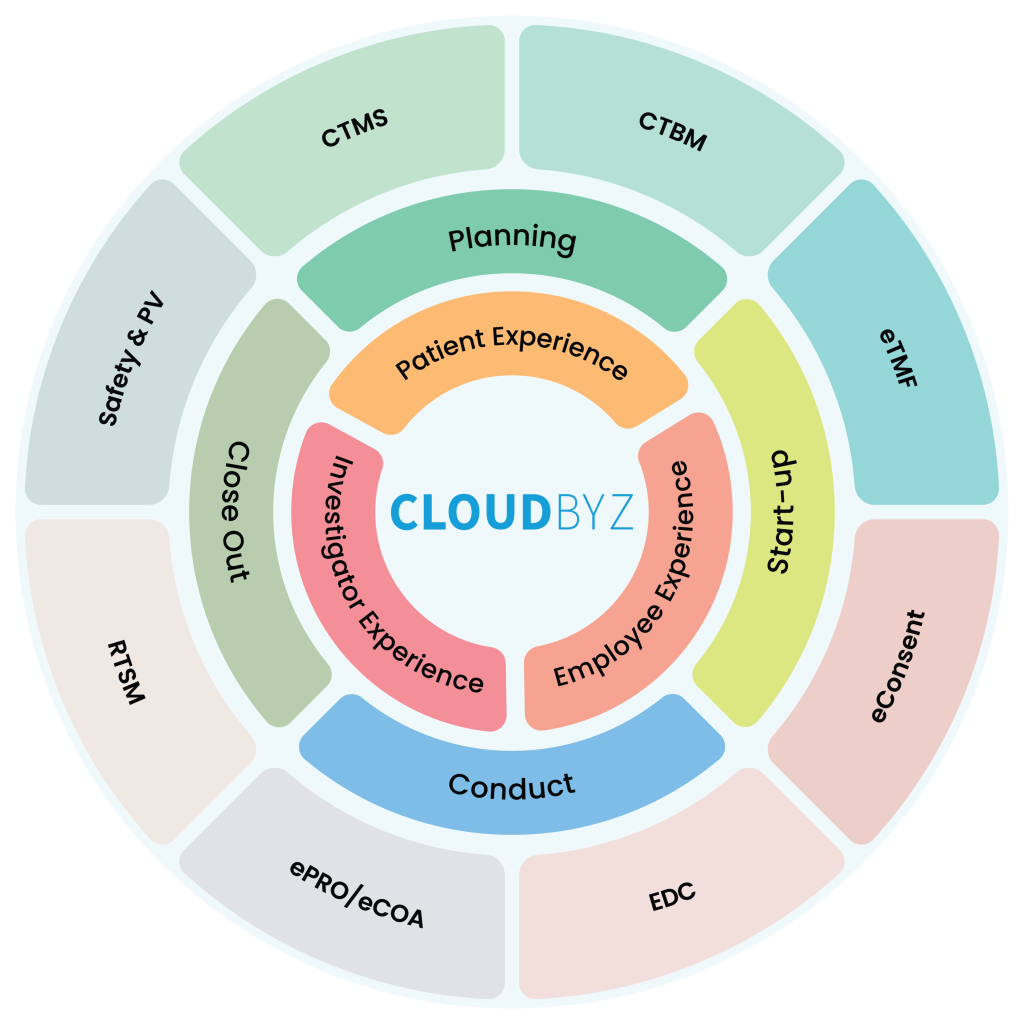
Cloudbyz’s unified clinical trial management solution covers the entire clinical trial lifecycle, from study planning to close-out. This all-in-one approach simplifies the management process and reduces the need for multiple tools or platforms. Cloudbyz solutions include Study Start-Up, CTMS, CTBM, eTMF, RTSM, EDC, eCOA, eConsent, ePRO, Safety & Pharmacovigilance. Key features include study planning, site management, patient recruitment, electronic data capture (EDC), monitoring, and reporting.
2. Real-time Visibility and Collaboration:
The platform promotes better decision-making and improved collaboration by providing real-time visibility into project status, study data, and key performance indicators (KPIs). By offering a centralized dashboard, study teams can quickly identify potential issues, address them proactively, and stay aligned throughout the trial process
3. Salesforce Scalable Platform Capabilities:
Cloudbyz leverages the power of the Salesforce platform to provide users with a seamlessly integrated solution. This enables organizations to take advantage of Salesforce’s platform capabilities, scalability, and flexibility. By consolidating clinical trial management with other Salesforce platform-based capabilities, users can enjoy a more cohesive and efficient experience
4. Customizability and Flexibility:
Cloudbyz’s platform is highly customizable, allowing users to adapt the solution to their specific needs and requirements. This flexibility ensures that organizations can tailor the platform to suit individual study protocols, workflows, and data management preferences, creating a more personalized and efficient user experience.
5. Enhanced Compliance and Security:
Built on the Salesforce platform, Cloudbyz’s solution adheres to stringent security measures and industry-standard compliance requirements such as HIPAA, GDPR, and 21 CFR Part 11. This ensures that sensitive data is protected and organizations can maintain regulatory compliance with confidence.
6. Streamlined Processes and Automation:
The platform simplifies clinical trial management by automating repetitive tasks, reducing manual effort, and streamlining processes. This leads to increased efficiency, cost savings, and faster trial execution. Features such as automated reminders, task assignments, and data validation ensure that study teams can focus on higher-level tasks and decision-making.
7. Robust Reporting and Analytics:
Cloudbyz’s solution offers powerful reporting and analytics capabilities, enabling users to gain insights, identify trends, and make data-driven decisions to optimize clinical trial performance. Customizable reports and dashboards allow study teams to monitor KPIs, track study progress, and assess the effectiveness of their strategies.
8. Scalability:
Designed to scale with the needs of its users, Cloudbyz’s platform can accommodate clinical trials of varying sizes and complexities. This ensures that the solution remains useful and valuable as organizations grow and evolve, meeting the ever-changing demands of the clinical research landscape.
9. Integration with Third-Party Tools:
Cloudbyz’s solution can integrate with a variety of third-party tools and applications, further enhancing its capabilities and streamlining the clinical trial management process. By connecting with tools such as electronic medical record (EMR) systems, laboratory information management systems (LIMS), and data visualization software, Cloudbyz’s platform can seamlessly incorporate additional functionality and data sources to provide a more comprehensive view of the clinical trial.
10. Exceptional Customer Support:
Cloudbyz is committed to providing exceptional customer support, ensuring that users have the assistance and resources they need to successfully implement and utilize the platform. From onboarding and training to ongoing technical support and regular updates, Cloudbyz’s dedicated customer success team works closely with clients to maximize the value they derive from the platform and address any challenges that arise.
11. Continuous Innovation and Improvement:
Cloudbyz continually invests in research and development to stay at the forefront of the clinical trial management software market. By incorporating user feedback, industry trends, and technological advancements, the platform is consistently updated and enhanced to deliver cutting-edge features and an ever-improving user experience.
12. Global Reach and Multi-Language Support:
Cloudbyz’s platform is designed to support clinical trials across the globe, with multi-language support and the ability to accommodate various regulatory requirements and standards. This enables organizations to manage global clinical trials with ease and ensures consistency in data management and reporting, regardless of location.
13. Cost-Effective Solution:
By offering a comprehensive and scalable solution, Cloudbyz helps organizations reduce the total cost of ownership for their clinical trial management software. The platform’s flexibility and adaptability, coupled with the elimination of the need for multiple tools or platforms, result in significant cost savings and a more efficient use of resources.
Conclusion:
Cloudbyz‘s Unified Clinical Trial Management Solution on Salesforce provides an unparalleled combination of features and benefits that revolutionize the way organizations manage clinical trials. By leveraging the power of Salesforce, focusing on key value propositions, and offering additional benefits like seamless third-party integrations, exceptional customer support, and continuous innovation, Cloudbyz is poised to make a significant impact in the clinical research industry. Embrace the future of clinical trial management with Cloudbyz and experience the benefits for yourself.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com


