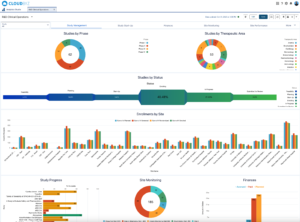
Clinical Trials Operations Oversight and Key Metrics to Track
Effective oversight of clinical trials operations is essential for ensuring the success of clinical research. By closely monitoring key metrics and implementing best practices, researchers can conduct trials that are efficient, ethical, and compliant with regulatory standards. Leveraging technology and fostering collaboration among stakeholders further enhances the oversight process, ultimately contributing to the advancement of medical science and the development of new treatments.